Multiscale Water Management in Polymer Electrolyte Membrane Fuel Cells
Proton exchange membrane (PEM) fuel cells are efficient clean energy production devices, but their low power density and high costs hinder their widespread adoption. Efficient management of the water generated from electrochemical reactions in PEM fuel cells is critical to address the performance and cost issues.
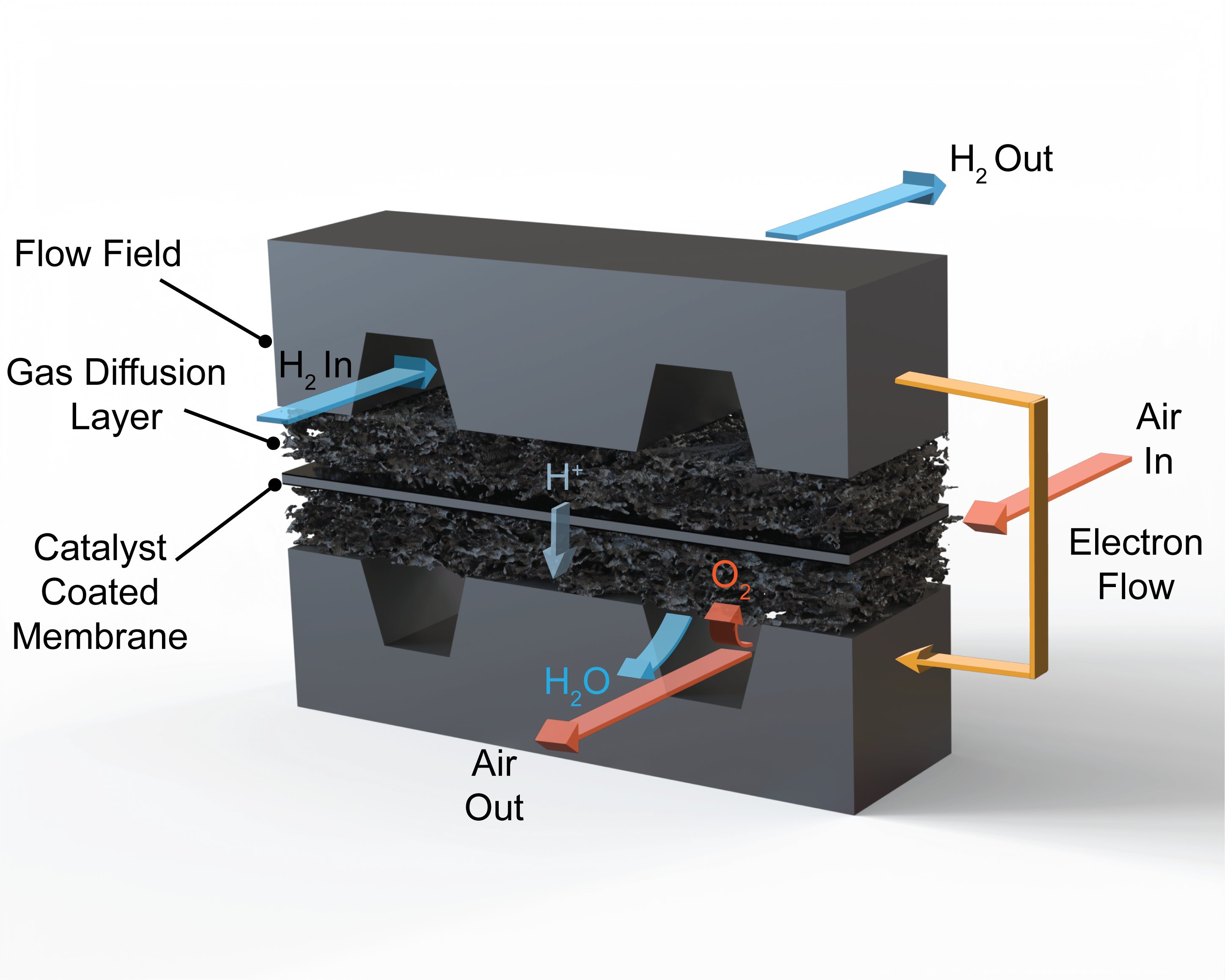
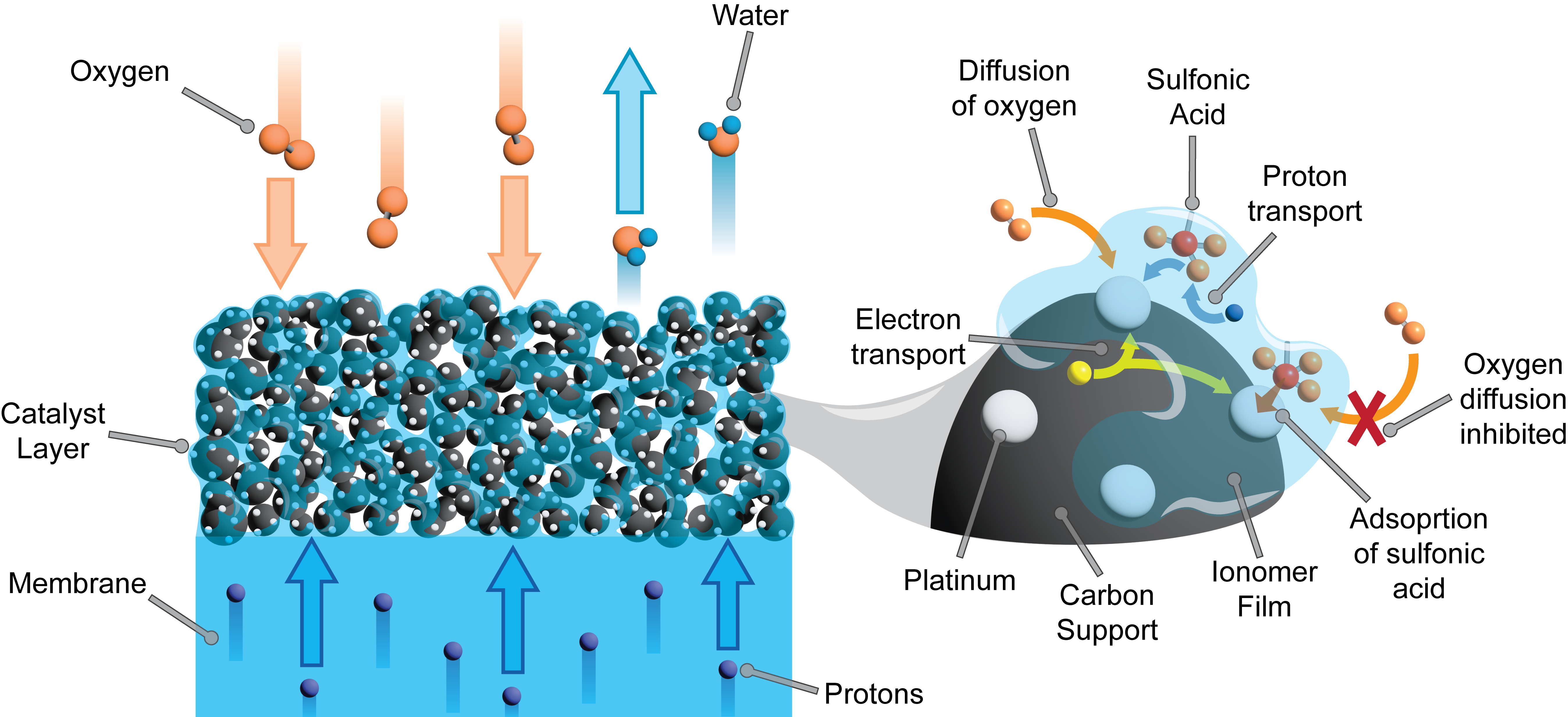
PEM fuel cells consist of a proton conductive membrane (typically Nafion®) surrounded by platinum-based catalyst layers that facilitate the hydrogen oxidation and the oxygen reduction reactions. The catalyst layers in PEM fuel cells have a porous nanostructure which consists of carbon supported platinum nanoparticles dispersed in a proton conductive polymer (ionomer) matrix. The catalyst layers are supported by microporous gas diffusion layers and flow fields that facilitate the transport of hydrogen and air to the reaction sites.
My Ph.D. research within the Bazylak group at the University of Toronto focuses on novel in-situ and operando X-ray characterization of PEM fuel cells and investigating novel materials for efficient simultaneous transport of liquid water and oxygen in PEM fuel cells.
Effects of flow field channel aspect ratio on advective transport
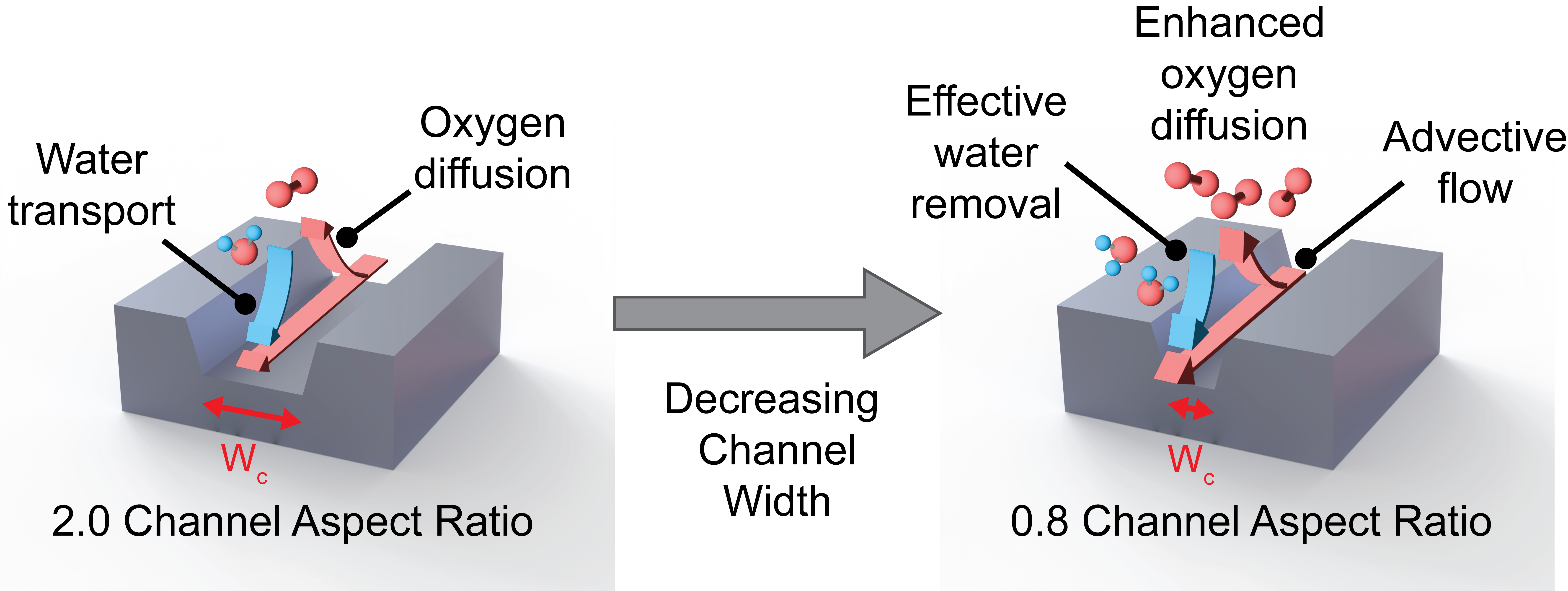
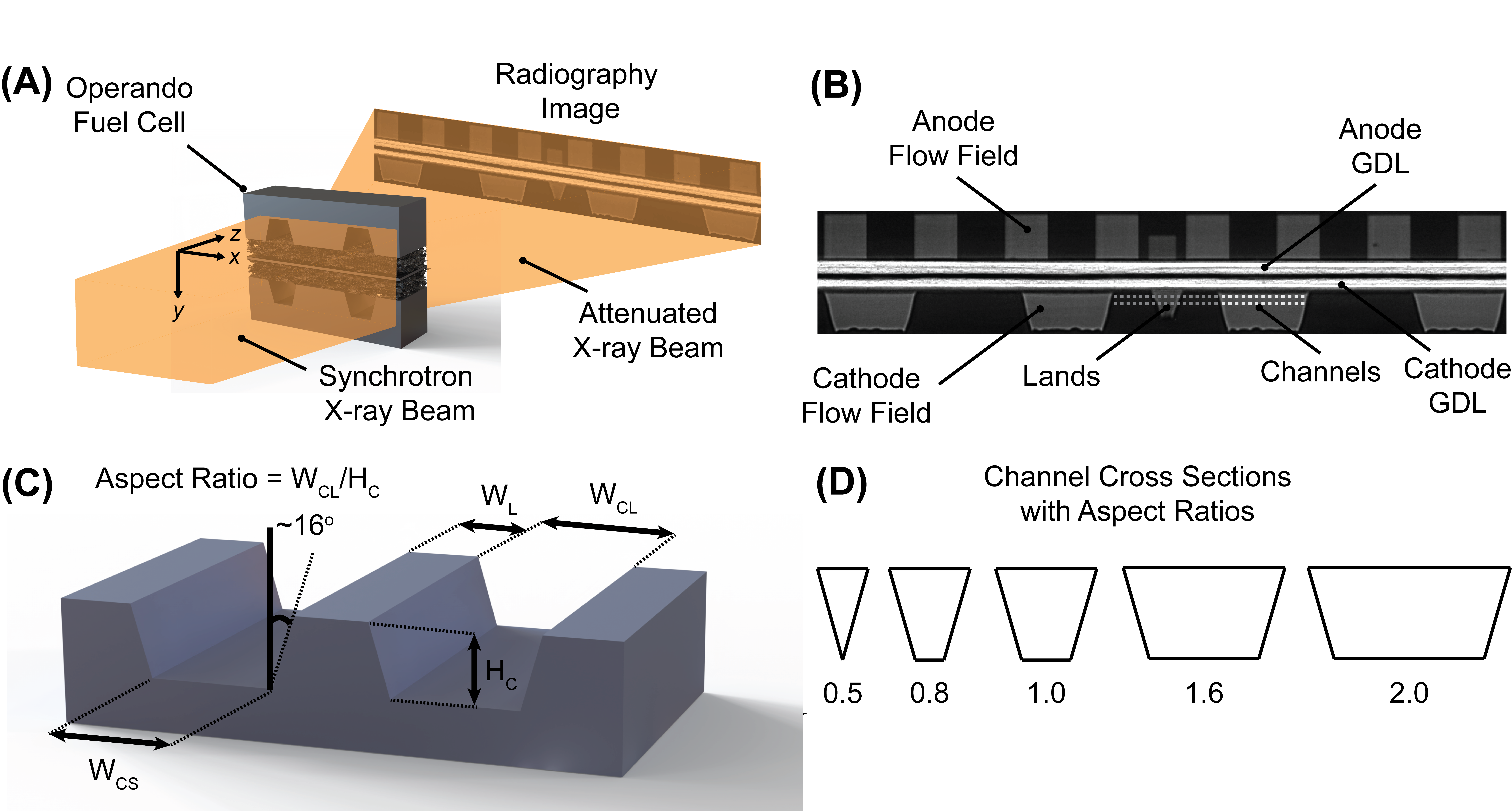
- In this work, we characterize the effects of decreasing the flow field channel aspect ratio on the performance of PEM fuel cells. Numerical studies have shown an enhancement in fuel cell performance at smaller channel aspect ratios; however, the operando liquid water accumulation in the gas diffusion layer and two phase pressure frop have not been characterized.
- We observed that a channel aspect ratio of 0.8 led to a 25% enhancement in peak power density with minimal pressure drop penalties. Decreasing the channel aspect ratio to 0.5 led to a 2 fold increase in the two-phase pressure drop in the channels compared to the 0.8 aspect ratio.
- For more details, please refer to the complete manuscript submitted to Green Energy and Environment in December 2025.
A natural gas diffusion layer for high performance PEM fuel cells
- Organized microstructures in conventional carbon paper gas diffusion layers have improved the mass transport performance of PEM fuel cells. However, the intrisic tortuosity of carbon paper hinders the enhancements compared to low tortuosity patterned microstructures developed from bottom-up approaches.
- Natural basswood has been used in thermal desalination for efficient water management due its intrinsic organized microstructure. In this work, we used a fluorine-free carbonized wood gas diffusion layer to improve the power density by 66% due to effective liquid water removal from the PEM fuel cell at 100% relative humidity.
- Low tortuosity organized microstructures are the key to next-generation high performance PEM fuel cells
Nanoscale in-situ hydration of polymer electrolyte membranes
- In this work, we quantified the in-situ nanoscale hydration of polymer electrolyte membranes infused with amorphous silica nanoparticles. Scanning transmission X-ray microscopy and Near Edge X-ray Absorption Fine Structure spectroscopy was used to characterize the liquid water accumulation in membrane as a function of relative humidity.
- The silica nanoparticles improved the membrane proton conductivity above 75% relative humidity due to the effective Grotthuss transport of protons.
- Differential scanning calorimetry highlighted that the proton conductivity enhancements resulted from a higher content of free water in the silica infused membranes.
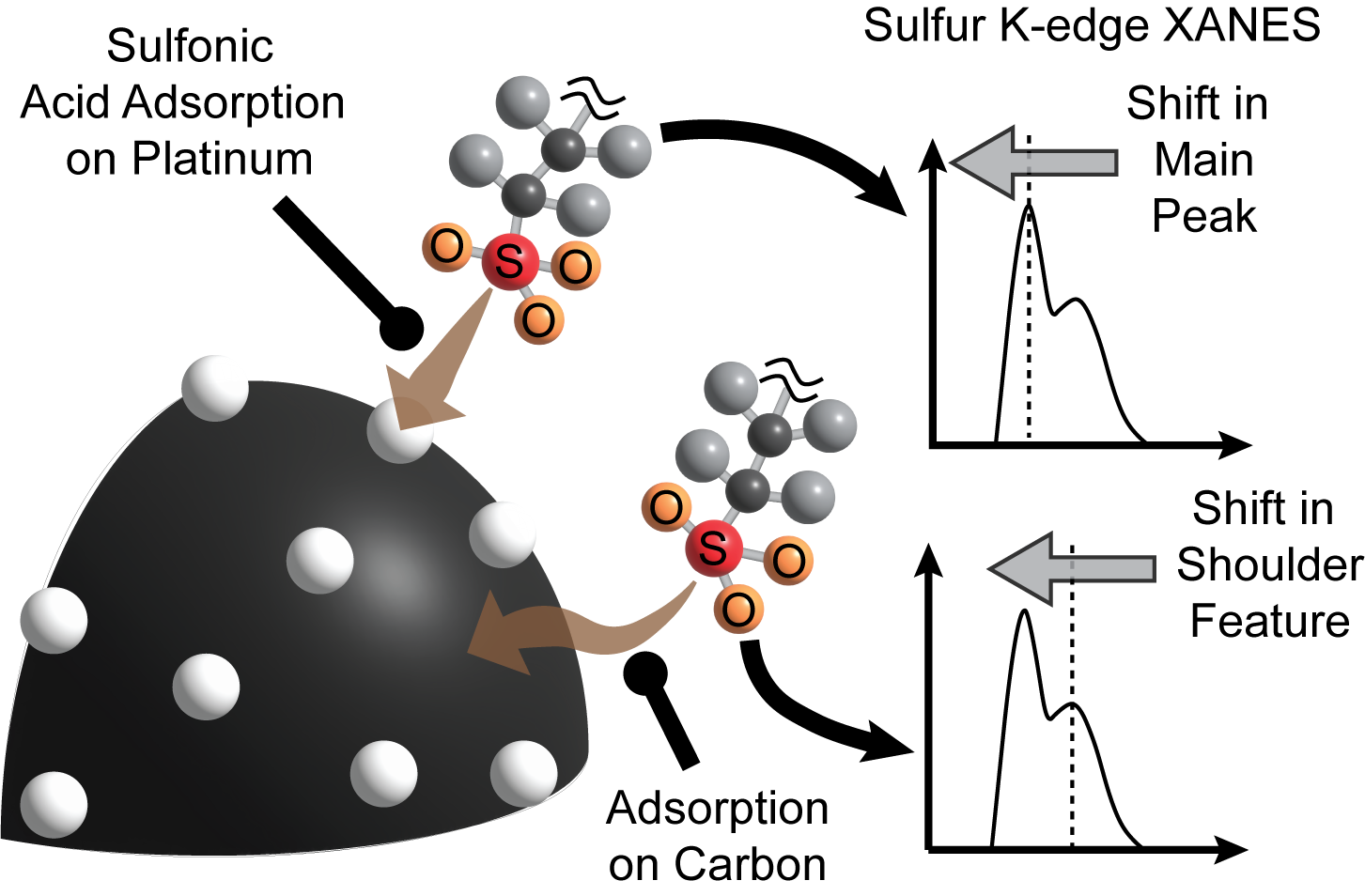
Characterizing sulfonic acid adsorption in PEM fuel cells
- Scanning transmission X-ray microscopy and NEXAFS spectra at the sulfur K-edge provided novel insights into the nanoscale sulfur poisoning mechanisms in PEM fuel cell catalyst layers. The chemical shifts in the main peak of the sulfur K-edge spectra of ionomer characterize the reduction of pristine sulfonic acid groups upon adsorption with platinum.
- The adsorption of sulfonic acid groups onto the catalyst occurs at high platinum concentrations (>20 wt.%) in the catalyst layer and leads partial sulfur oxidation states ranging from 4.8 - 4.9.
- The intensity and excitation energy of the shoulder feature in the sulfur K-edge spectra of ionomer revealed novel insights into the chemical environment of sulfonic acid groups in the catalyst layer.